Applications
Undetected cold chain failures are a growing problem affecting various market sectors. However, it is in the pharmaceutical and healthcare industry where product degradation can have the most critical impact. Annually 20–25% of vaccines, biopharmaceuticals and essential medicines are damaged in transit due to equipment failures, improper use of coolants and transportation delays, leading to losses exceeding €29bn.
The market increasingly calls for more advanced and reliable cold-chain monitoring solutions to address a growing demand and emerging challenges such as:
- Increased transparency of temperature-controlled supply chains for recall management and logistics optimisation.
- Sustainable and economical monitoring at item-level or for last-mile distribution.
- Reliable monitoring for deep-frozen cold chains, driven by the emergence of new classes of vaccines and biologics.
- Intuitive, consumer-facing monitoring solutions for self-administered medications and domestic healthcare.
VERITASCAN time-temperature indicators are designed to provide a transformative and highly versatile solution that addresses the market requirements, as illustrated in the following Application Notes.
Application notes
- Traceable cold chain integrity and recall liability
- Medical inventory management
- Ultra-low temperature cold chain monitoring
- Domestic use by consumers
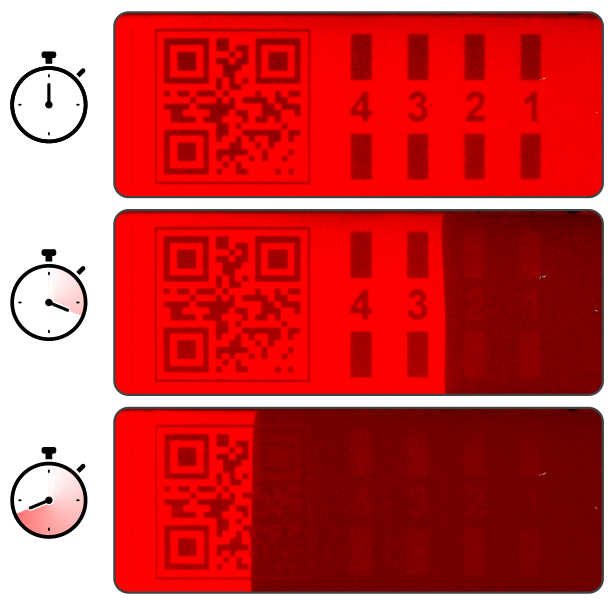
Traceable cold chain integrity and recall liability
A key aspect of cold chain failures involves determining the liability for recall costs. With the increasing complexity and diversity of supply chains, how can logistics providers prove cold chain compliance at destination or distribution exchange?
Indicators featuring temperature-erasable scannable codes specific to the logistics provider or shipment ID can be used to certify compliant shipment. Picture a relay race: logistics company A hands over the shipment to company B. The code is scanned with location and time stamps. No scannable code – no acceptance at transfer point or destination, with transparent indication of recall liability.
Enabling features of VERITASCAN: (i) temperature-erasable and serialisable scannable codes, and (ii) on-demand indicator activation.

Medical inventory management
Temperature-sensitive medicines such as oxytocin and insulin are routinely taken out of chilled storage in hospitals to be warmed to room temperature and at-hand in the treatment areas. The durations of medical procedures are unpredictable and can last >10 h. How can the hospital staff decide whether the medicine can be placed back into chilled storage and safely used again?
Indicators can be applied to common medicines to record cumulative exposure to heat during routine procedures, registering the residual shelf life. This ensures efficient inventory management and reduces healthcare spending on wasted medicines.
Enabling features of VERITASCAN: (i) small size, (ii) low item-level cost and (iii) progressive exposure scale.
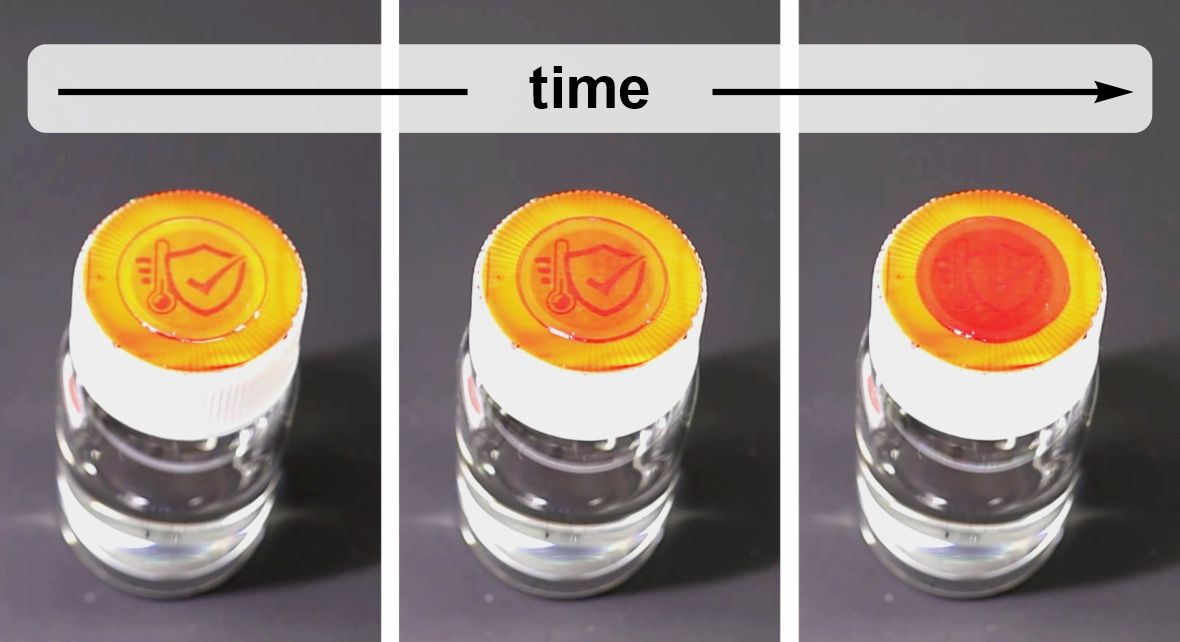
Ultra-low temperature cold chain monitoring
Numerous products require storage substantially below –20 °C, down to –70 °C (Fig. 4(a)), which are temperature not covered by existing TTIs. How can a healthcare provider know that (i) the items were stored at cryogenic temperatures and (ii) are warmed to room temperature before use?
Vial-cap TTIs activated at point of shipment featuring an intuitive pictogram reading can highlight correct ultra-cold storage and subsequent thawing prior to use.
Enabling features of VERITASCAN: (i) threshold temperatures below –20 °C, (ii) clear SAFE/UNSAFE reading and (iii) minimal waste footprint.
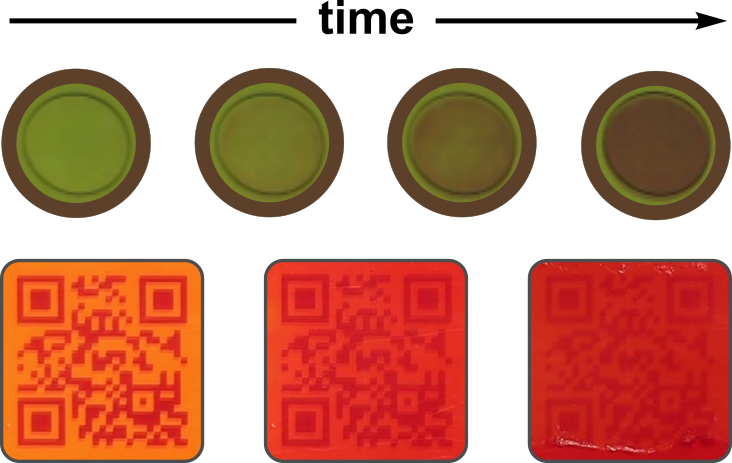
Domestic use by consumers
Numerous pharmaceutical, dietary and cosmetic products intended for domestic use require chilled storage. How can the consumer be informed and encouraged to store the product correctly?
Item-level indicators activated at point of sale such as the pharmacy can be attached as a label to the packaged product to inform the consumer of its integrity and upcoming expiry.
Enabling features of VERITASCAN: (i) clear and intuitive SAFE/UNSAFE reading, (ii) low item-level cost and (iii) minimal waste footprint.